– In six months, we will probably have the first Neuralink integrated into a human.
This is how Elon Musk opened the press conference about Neuralink, from the headquarters in Fremont, California.
By drilling a hole in the skull and directing electrodes to different parts of the brain, Neuralink will turn science fiction into reality.

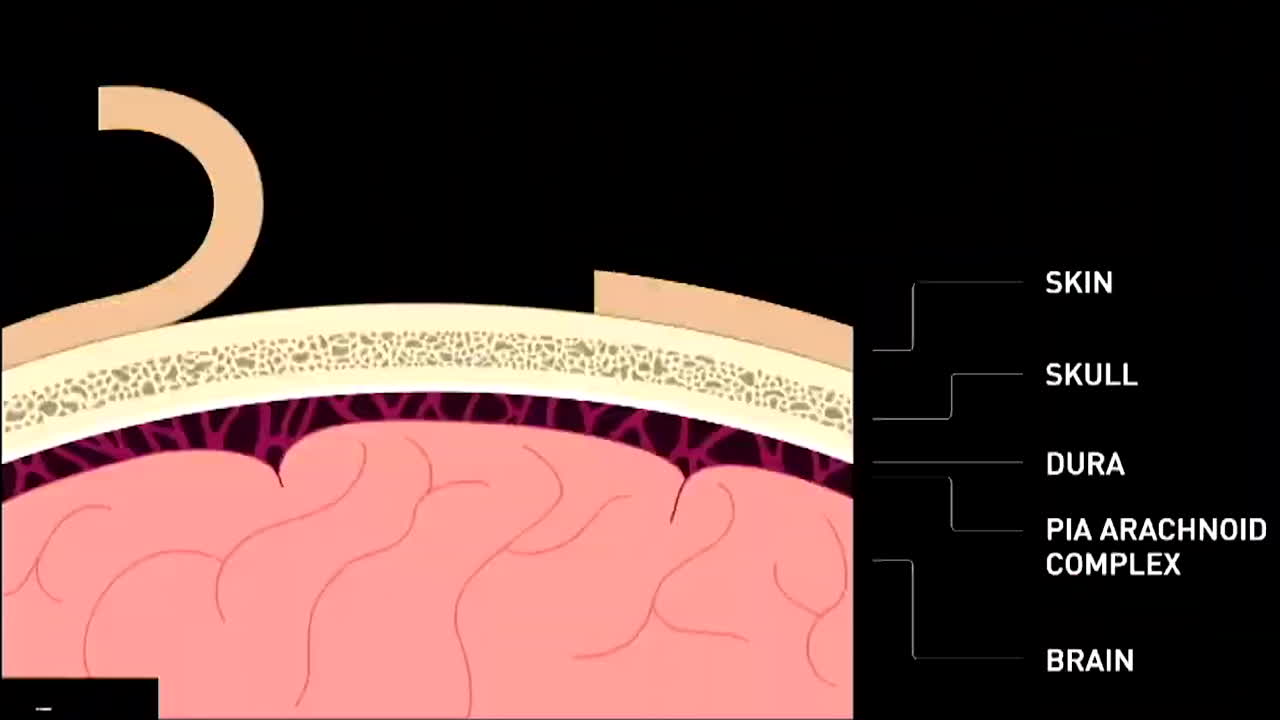
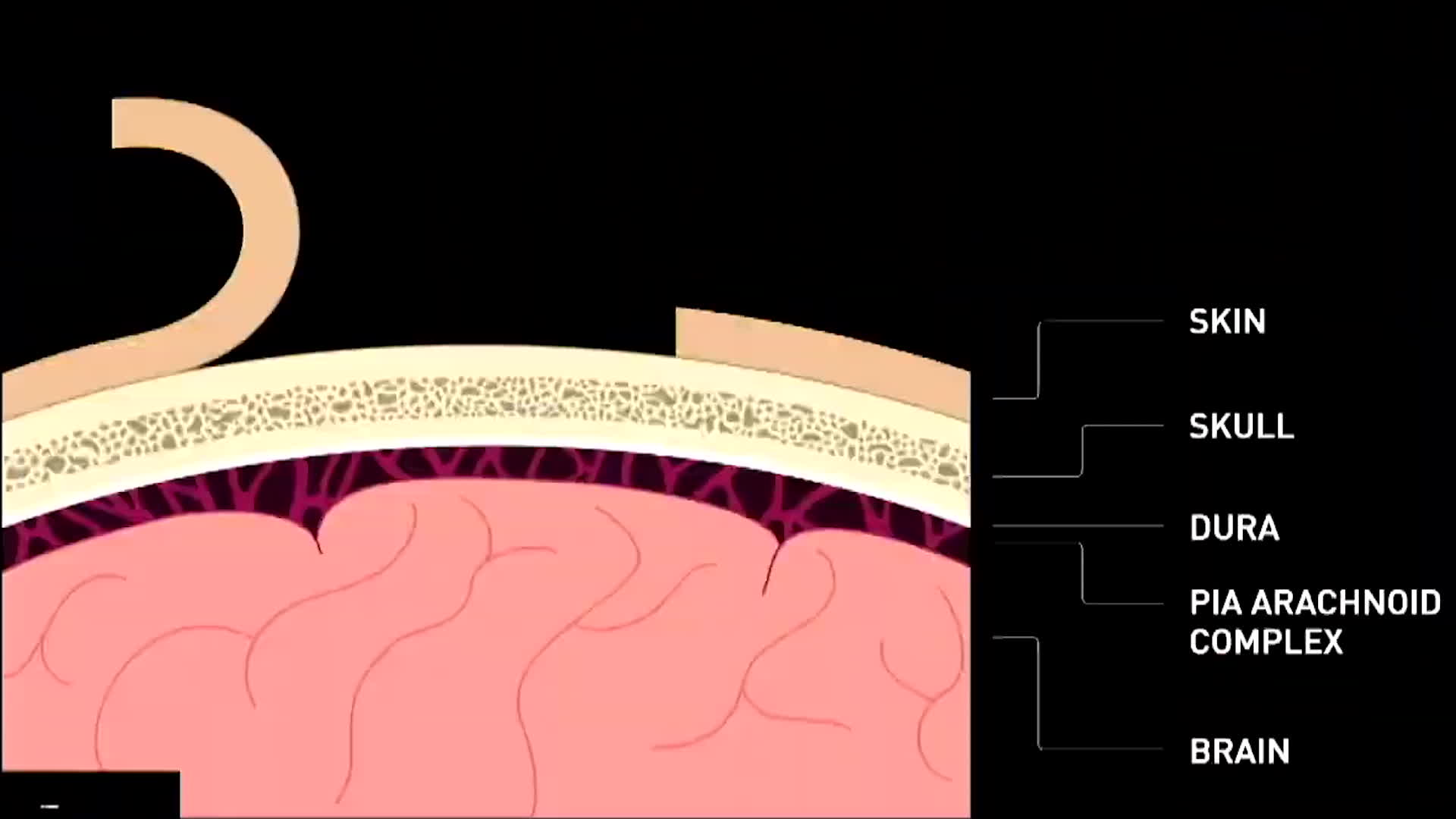
A video animation of the operation was shown at the press conference.
Neuralink
The ambition should be to be able to help people with neurological injuries. For example, returning control of one’s body to the disabled, or returning sight to the blind.
Musk said optimistically: – Even where no one has ever seen, where he was born blind, we believe we can restore sight.
– This is not a chip that scans brain waves, but it helps control brain waves, continued from the stage.

From a press conference in 2020: Elon Musk with a prototype of the device that will install chips in the brain.
Photo: AFP
Monkeys play computer games
In recent years, Neuralink has conducted large-scale animal trials.
Among other things on monkeys, which have a Neuralink chip installed.
In videos shown at the event, the monkeys can be seen controlling cursors on a screen and using a keyboard, using their brain waves.
In addition, Neuralink has previously demonstrated how a monkey can play the computer game Pong using a brain chip.



The monkey plays Pong in the Neuralink experiment by thinking about where the ball will go.
Neuralink
However, this did not happen without problems. Earlier this year, Elon Musk was able to confirm that one of the monkeys died as a result of the project.
A wonderful revolution
Matthias Toft is the Department Head and Senior Physician in the Department of Neurology at the University Hospital Oslo. He refers to the technology as “exciting” and thinks it’s realistic that it could soon become a reality.
– If you can make this business a success, that’s a great revolution, he says.
He explains that similar projects involving connecting the brain to, among other things, computers and phones have previously shown the project to be feasible in principle.
By this I mean that thoughts can control something outside the body through such communication.
– If you can make it work well enough to convey enough information and become accurate enough, it offers a number of exciting possibilities for medical treatment of paralysis, speech disorders, blindness and many other serious problems, he continues.

Neurologist Matthias Toft describes the technology as a remarkable revolution. He explains that people with paralysis can control various devices using the power of thought, and this will open up many different areas of use.
Photo: Stein Schenstadt
Despite the fact that he is positive about the technology, he nevertheless understands that the project does not come without challenges.
– All new therapies will have, among other things, ethical issues and security challenges. There would also be a risk when electrodes were implanted in the brain, which could cause complications such as bleeding and infections, Toft explains and adds that electrodes are already implanted in the brain today to treat, among other things, Parkinson’s disease.
The Food and Drug Administration is in power
Although Musk hopes the brain chip could be tested in humans within six months, it must first be approved by the US Food and Drug Administration (FDA).
The agency is under the US Department of Health and Welfare, and regulates, among other things, the entry of drugs and medical equipment into the United States.
The Food and Drug Administration (FDA) will have to go through a full assessment of the potential risks that the procedure may have, and therefore assess whether the chip is safe to use.
We’ve submitted most of the paperwork to the FDA, Musk said at Thursday’s press conference.

“Explorer. Unapologetic entrepreneur. Alcohol fanatic. Certified writer. Wannabe tv evangelist. Twitter fanatic. Student. Web scholar. Travel buff.”




