published
March 5, 2024
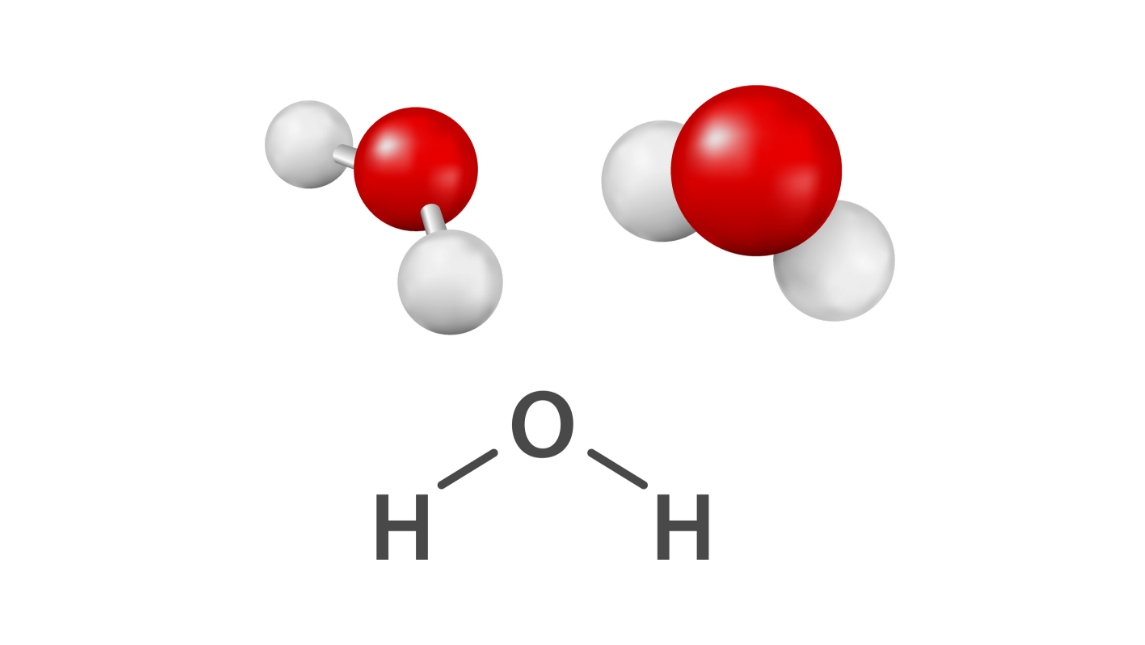
Nearly a hundred years ago, it was possible to see the structure of molecules using a method called electron diffraction. Therefore, most chemists felt confident in the existence of these structures. Most people who studied chemistry in high school have seen textbooks full of molecular structures.
Electron diffraction
Electron diffraction is a method used, among other things, to study molecular structure. The method works by firing electrons at the molecule to be examined.
When an electron hits a molecule, it changes direction. By firing many electrons at the molecule, a pattern is formed of where something is “solid.” By interpreting the pattern that forms, researchers can say something about the distance between atoms. So they also understand what the molecule looks like.
But since molecular structure conflicts with quantum mechanics, there has been debate within the chemistry community. According to quantum mechanics, it is not certain that these structures exist. Part of the reason for this is that it has not been possible to use quantum mechanics to find molecular structure – even though all chemists believe it should be so. Another reason is due to quantum mechanics itself:
– It is a contradiction that we can see the molecular structure, while the nature of quantum mechanics shows that we cannot know anything about the position of particles, while we know the speed, explains Thomas Bondo Pedersen, professor of theoretical chemistry at the University of Oslo.
Molecular structure is a contradiction
According to him, quantum mechanics cannot distinguish between identical particles, for example protons in a water molecule. They are all the same and nothing can be said about a specific proton in a water molecule for example. Pedersen therefore believes that the molecular structure is paradoxical, because it is then possible to know where the individual molecules are located.
Quantum mechanics
Quantum mechanics is several theories that describe some properties of fundamental structures, such as electrons, protons, atoms, and molecules.
Quantum mechanics describes how they are built, how they move, and how they interact with each other.
Pedersen says this problem was something Robert Oppenheimer — yes, the Oppenheimer made into a movie — and Max Born tried to solve in 1927. The result was called the Born-Oppenheimer approach. This approach assumes that particles with greater mass move more slowly than those with less mass.
Since atomic nuclei are several thousand times heavier than electrons, they can be assumed to have a fixed position with respect to the electrons. With this approach, Born and Oppenheimer were able to force quantum mechanics to include the concept of molecular structure.
Mathematicians have since shown that this is a very good approximation, even though it violates very fundamental laws of nature for identical particles. This approach has been widely used by physicists and chemists because it is easier to calculate than full quantum mechanics. Because theoretical calculations always agree very well with experimental investigations. So, it should really be possible to go the other way as well. That is by saying; Finding the structure of a molecule using quantum mechanics without using the Born-Oppenheimer approximation. This has been a problem in theoretical chemistry for a long time, but now someone has solved it correctly:
-We have now proven that the method of Born and Oppenheimer is correct. We have shown that it is possible to go in the other direction, from quantum mechanics to molecular structure, says Pedersen.
The molecular structure we have seen so far is merely an explanation
Pedersen explains that the way electron diffraction works is part of the reason for the uncertainty about the existence of molecular structure. What scientists see is not the atoms themselves, but the way the electrons are scattered.
The way we explain this spread depends on theory, and this is the theory we have arrived at, Pedersen explains. We then use theories to create the models and calculations necessary to explain experimental observations.
However, he confirms:
– We have always believed that molecular structures exist, because there are many things that indicate this, he believes.
Powerful computers were needed to solve the problem
Part of the reason the structures have finally been proven only now is partly due to more powerful computers and better calculations, he explains.
Pedersen says he has a one-year research appointment to study how molecules behave when affected by very short laser pulses. So you can't use the Born-Oppenheimer approximation, and the question of molecular structure in quantum mechanics becomes very central. Things only accelerated when he reached out to Lukas Lange, who was a postdoctoral fellow at the Hilleras Center and is now a junior professor in Berlin. Like Pedersen, Lang believes that molecular structures exist, and that they can be found using quantum mechanics.
Using a method to find the probability of different types of particles being relative to each other, they were able to create a kind of map. For example, the researchers used one of the most common molecules in the universe, called D3+where Ludvik Adamowicz of the University of Arizona was able to provide the complete quantum mechanical probability distribution.
However, there was one problem.
In theory, the particle could be anywhere in the universe
– One particle could be on the Moon, and another could be on Earth. The problem is that particles in the same molecule are not particularly likely to have such a position relative to each other. Therefore, we had to find a way to find only relevant locations – the calculation becomes very large if we have to include all points in the universe, Pedersen explains.
– If we choose the points randomly, we will get a number of insignificant points, explains Henrike Moseli-Cesar, a postdoctoral fellow in theoretical chemistry at the University of Oslo. Therefore, we had to find a way in which we could determine the points using the distribution.
The approach they used to eliminate unexpected situations is called Monte Carlo selection, and it's something Cesar is an expert at. In this way, they get an overview of what they call the prevailing probabilities.

Mathematics used in “space travel”.
Now researchers have obtained the points in the universe that have the highest probability of a particle being present in a molecule. It then remains to see how these points relate to each other:
“We then used the direction vector, which is actually used to calculate motions in space,” explains Pedersen
This is how they obtained a group with different structures. Pedersen explains that there's a good reason for this:
– Molecules rotate and vibrate, which is why we get a class with different structures.
It's never been offered before – but the time has come
However, they did find quite a few structures. Therefore, researchers had to classify them. Lang is an expert in machine learning. Using machine learning algorithms, he found a way to classify and sort different structures.
Then they saw that there was a more or less similar structure everywhere, which was in fact similar to what was observed. The difference between the different structures was primarily that they were oriented differently in space. They thus showed that it is possible to find molecular structure using quantum mechanics.
This has never been proven possible, but the time has come, says Pedersen.
He continues with a smile:
Now, most chemists, if they think about it, can sleep well without having nightmares, he says.
And if anyone is wondering why the law of quantum mechanics that states that particles cannot be fixed is not broken in the new way:
– The laws of quantum mechanics still apply, because our method does not identify individual particles. We use the probability distribution as the basis for the structure, while Born and Oppenheimer placed the protons in specific locations. There's a subtle difference, Pedersen says.
source:
Lukas Lange, Henrik M. Cesar, Lodwik Adamowicz, and Thomas Bondo Pedersen, Quantitative definition of molecular structureJournal of the American Chemical Society, January 2024

“Explorer. Unapologetic entrepreneur. Alcohol fanatic. Certified writer. Wannabe tv evangelist. Twitter fanatic. Student. Web scholar. Travel buff.”